The First Step In Taking Care Of Your Health
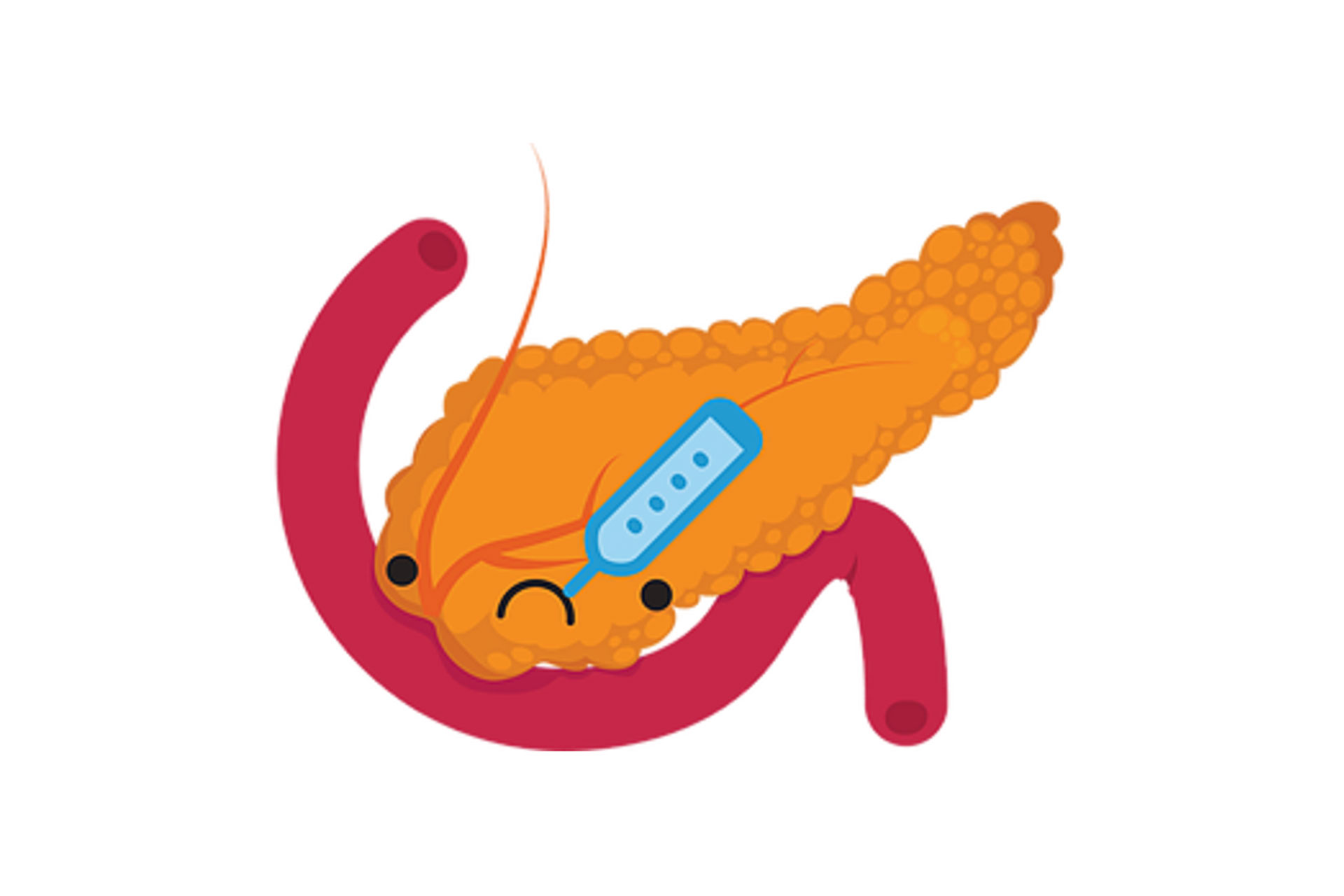
Understanding the use of magnesium bicarbonate begins with understanding the pancreas, which is the organ most responsible for providing the bicarbonate our bodies need. The pancreas is a long, narrow gland, which stretches from the spleen to about the middle of the duodenum. It has three main functions. Firstly, it is to provide digestive juices, which contain pancreatic enzymes in an alkaline solution to provide the right conditions for the digestive process to be completed in the small intestines. Secondly, the pancreas produces insulin, the hormone that controls blood sugar by the metabolism of sugar and other carbohydrates. Thirdly, it stores and secretes bicarbonate salts to neutralize acids coming from the stomach to provide the right environment for the pancreatic enzymes to be effective. These bicarbonate salts also act as buffers to maintain the normal levels of acidity (pH) in blood and other fluids in the body.The bicarbonate buffering system is, functionally, the most important buffering system in the body. The body has a number of ways it synthesizes and obtains its bicarbonates.
Biology, Biochemistry, and The Carbonic Acid/Bicarbonate Buffer System of the Blood
Carbon dioxide (CO2) in the blood reacts with water (H2O) in red cells resulting in the production of carbonic acid (H2CO3). This is known as the carbonic acid/bicarbonate buffer system of the blood. Bicarbonate accounts for the transport of 60% to 70% of carbon dioxide in the blood. Carbonic acid is stable at 4o Celsius; therefore, it immediately dissociates a hydrogen ion (H+) and becomes H+ and a bicarbonate ion (HCO3). An enzyme present in red blood cells, carbonic anhydrase, catalyzes the reaction of water and carbon dioxide to form bicarbonate ions. Carbonic anhydrase also loses a hydrogen ion to become a bicarbonate ion. The H+ subsequently binds to hemoglobin; this binding triggers the Bohr effect.
What this means is that as the amount of CO2 increases, more H+ are formed and the pH will decrease. Thus, a lower pH in the blood is suggestive of an increased CO2 concentration, which in turn is suggestive of a more active tissue that requires more oxygen. According to Bohr, the lower pH will cause hemoglobin to deliver more oxygen!
H + HCO3 <=> H2CO3
The bicarbonate part (HCO3) easily associates with H+ ions of any acid and the bicarbonate ions associate back to carbonic acid. If too many H+ build up, the equation above will be pushed to the right, and bicarbonate ions will absorb the H+ to form carbonic acid. Similarly, if H+ concentrations drop too low, the equation will be pulled the left and carbonic acid will turn into bicarbonate, donating H+ ions to the solution. When this buffer system is compromised, the body’s pH becomes acidic and the body starts to pull minerals out of the bones and/or put the liver into an ammonia cycle to handle the acid overload.
The bicarbonate ions defuse out of the red blood cells to be carried by the plasma; again, the liquid portion of blood. It’s what suspends red blood cells, platelets, and other cells within our bloodstream. It also contains thousands of different proteins and other substances (electrolytes for example) that are needed for our bodies to function normally. Like bicarbonate ions in river water, bicarbonate ions in plasma are unstable, and they stabilize themselves with calcium, magnesium, sodium, and potassium hydroxides found in the plasma. Electrolyte salts may enter or leave the cell membrane through specialized protein structures embedded in the plasma membrane called ion channels. The pancreas is a reservoir for these bicarbonate salts and it excretes them when needed to maintain the pH balance of our body fluids and aid in digestion. There are two other important buffer systems in our bodies:The Phosphate Buffer System
Phosphoric acid changes pretty quickly into dihydrogen phosphate. Dihydrogen phosphate is an excellent buffer, since it can either grab up a hydrogen ion (H+) and reform phosphoric acid, or it can give off another hydrogen ion and become monohydrogen phosphate (a weak base). Therefore, the phosphate buffer system can accept or donate hydrogen ions depending on the solution it is in.
The Protein Buffer System
Proteins themselves can act as buffers. When bicarbonate ions form in the blood, hydrogen ions are produced; blood proteins absorb hydrogen ions.Amino acids can accept or donate hydrogen ions, making them excellent buffers. And any given protein typically has hundreds of amino acids. So, proteins make superb buffers.
Metabolic acidosis is characterized by a primary reduction in serum bicarbonate along with a decrease in pH. One or more of the following may account for it:Poorly Functioning Kidneys:
Inability of the kidneys to excrete the metabolic acidsThe kidneys are not generating sufficient bicarbonateExcessive loss of bicarbonate via kidney or gastrointestinal tract.
Poorly Functioning Liver:
The liver is important in acid-base physiology and this is often overlooked. It is important because it is a metabolically active organ which may be either a significant net producer or consumer of acids. The acid-base roles of the liver may be considered under the following headings:Complete oxidation of carbohydrates and fat, which occurs in the liver, produces carbon dioxide but no fixed acids. As the liver uses 20% of the body’s oxygen consumption, this hepatic metabolism represents 20% of the body’s carbon dioxide production; as the carbon dioxide diffuses out of the liver it helps sustain the carbonic acid/bicarbonate buffer system of the blood.The metabolism of various organic acids in the liver results in consumption of H+ and regeneration of the extracellular bicarbonateMetabolism of ammonium to urea (a weak base). Our bodies cannot tolerate high concentrations of urea, however, it is less toxic than ammonia and urea is removed efficiently by the kidneysExcess Production of plasma proteins.
Increased Production of Fixed Metabolic Acids:
Over exercising – production of excess lactic acidCatabolism of excess proteinsExcessive stress can increase the production of acidsKetoacidosis (over production of ketoacids) the pancreas is producing insufficient insulin to slow this production and/or carbohydrate stores are inadequate or the body cannot use available carbohydrates as a fuel.Fasting, particularly if prolonged, can lead to mild ketoacidosis.
Net Addition of Strong Acids:
Consuming acid rain (sulfuric acid [H2SO4] nitric acids [HNO3]) mitigates the carbonic acid/bicarbonate buffer system of the blood. (Not to mention the hydrofluorosilicic acid [industrial waste fluoride], hypochlorous and hydrochloric acids [chlorine reactions], haloacetic acid [chlorine and bromine reactions], and ido-acids [chloramine reactions] in our urban water supply).
Natures equation: H2O + CO2 <=> H2CO3 <=> H+ and bicarbonate (HCO3)New deadly equation: H2O + H2SO4 + HNO3 + CO2 => acids
Acid-producing diets i.e. processed foods like white flour, sugars, high fructose corn syrup, meats, grains, beans, nuts, and dairy productsOvereating, too much alcohol, tobacco, chemicals, carbonated drinks, coffee, etc.Salicylic acid found many vegetables, fruits, nuts, herbs, and seasonings (primary active ingredient in aspirin)Toxic alcohols, including methanol, ethylene glycol, diethylene glycol, and propylene glycol (found in many consumable products)Aspartame — the methanol carbon of aspartame converts to formaldehyde and then to formic acid. Formic acid it the toxic methanol metabolite that can contribute to metabolic acidosis. Methanol toxicity mimics, among other conditions, multiple sclerosis and systemic lupus. Aspartame can change chemical levels in your brain that affect behavior, and human studies reported depression, menstrual irregularities, constipation, headaches, and fatigue as common side effects! Aspartame is in over 5,000 products.Acetaminophen (Tylenol), formic acid, glycolic acid, and oxalic acid (found in many consumable products)Many prescription and over-the-counter drugs contribute to an acid-forming environment.
Failure to Ingest Sufficient Bicarbonate Salts via Drinking Water.
In the majority of cases of metabolic acidosis increased acid production and the decreased ability to buffer the acids are mainly due to dietary habits and contaminated dead water. The elimination or control of the underlying cause is obviously a high priority in the treatment of all forms of metabolic acidosis.
Dr. Robert Young, states, “Excess acidity is a condition that weakens all body systems. The pancreas is one of our body’s organs charged with the awesome responsibility to alkalinize us.”The pancreas, an organ largely responsible for pH control, is one of the first organs affected when general pH shifts to the acidic. A highly acidic pH level puts the pancreas, liver, and all the body’s organs at risk. When our pH levels are imbalanced we cannot effectively assimilate vitamins and minerals.
Our blood pH has a very narrow range of around 7.35 to 7.45. If our blood’s pH deviates from this range, we will be sick or have symptoms of falling sick. If it falls below 6.8 or above 7.8, our body cells will stop functioning and death will occur.
Metabolic acidosis—acute or chronic—can have considerable adverse effects on cellular function and can contribute to increased morbidity and mortality.
Bicarbonate generation is stimulated by a high protein diet and exercise. However, metabolic acidosis—acute or chronic overwhelms the pancreas’ ability to operate effectively. With excess acids in the body the pancreas cannot store or secrete enough bicarbonate salts to neutralize the acids and balance our pH. Without sufficient bicarbonate salt reserves, the pancreas is slowly destroyed; the body is not able to maintain its normal pH levels. The body is now forced to pull calcium, magnesium, potassium, and sodium from the bones to counteract the acids and keep the pH of our blood in check. If this process is not sufficient the liver goes into ammonia cycle to neutralize the acids. Metabolic acidosis—acute or chronic, which manifests as depleted alkaline (bicarbonate salt) reserves, is the underlying cause of much of human disease.
Although acute metabolic acidosis affects a number of organ systems, animal studies suggest that it affects the cardiovascular system most critically. Adverse effects primarily include decreased cardiac output, arterial dilatation with hypotension, altered oxygen delivery, decreased ATP production, predisposition to arrhythmias, and impairment of the immune response. Mental confusion and lethargy are often observed in patients, despite minor changes in cerebrospinal and brain pH. Lymphocyte function is suppressed, leading to increased inflammation and an impaired immune response. (Lymphocytes are any of the nearly colorless cells formed in lymphoid tissue, as in the lymph nodes, spleen, thymus, and tonsils, constituting between 22 and 28 percent of all white blood cells in the blood of a normal adult human.)The main adverse effects of chronic metabolic acidosis are increased muscle degradation and abnormal bone metabolism, as well as indirect effects on these tissues emanating from alterations in the secretion and/or action of several hormones. These abnormalities are more frequent and severe with greater degrees of metabolic acidosis, but even mild metabolic acidosis contributes to the development of bone disease and muscle degradation. Cellular energy production is compromised.
In addition, the cellular response to insulin is impaired, partly as a result of a pH-dependent decrease in the binding of insulin to its receptor, which plays a role in type 2 diabetes (the most common form of diabetes). Metabolic acidosis also causes brain damage and cerebral palsy in newborn babies.
When calcium, magnesium, potassium, and sodium bicarbonates are supplemented in the body they buffer excess acids; this allows the pancreas to store bicarbonate; the pancreas now has sufficient reserves to secrete bicarbonate when needed and keep our pH balanced. The most important effect of bicarbonate ingestion is the change in acid-base balance as well as blood pH and bicarbonate concentration in biological fluids.
As changes in extracellular and intracellular pH underlie the adverse effects of metabolic acidosis, the administration of base—primarily in the form of sodium bicarbonate—has been the mainstay of therapy. However, sodium bicarbonate administration has also been postulated to be a contributory factor in the development of cerebral edema in children with ketoacidosis. Furthermore, controlled studies of sodium bicarbonate administration were not shown to improve cardiovascular dysfunction in patients with acute metabolic acidosis. Moreover, consistent high intake of sodium increases risk of fluid retention and swelling of the extremities, or edema, high blood pressure (hypertension), heart or kidney disease. Consequently, there is disagreement among clinicians about the value of sodium bicarbonate administration in these acid-base disorders and criteria for the administration of sodium bicarbonate vary widely.
On the subject of salt, sodium chloride (table salt) is poison. Sea salt contains 84 inorganic minerals bound by sodium chloride. The electrolytes found in our blood closely resemble the ocean. We can live about forty days without food, about three days without water, about three minutes without oxygen, and about thirty seconds without salt. We should be on a no sodium chloride diet and a high sea salt diet (without added iodine)!
PARADIGM SHIFT
One study sponsored by the National Institutes of Health shows that 68% of Americans are magnesium deficient. Other experts put the number closer 90%. The first thing we should ask is, “why?” Here are a few of the facts.
The fertilizer used to grow our food (even organic) is NPK (nitrogen, phosphate, and potassium) and it is highly acidic, it kills the microbes in the soil. The plants can no longer convert inorganic minerals into organic plant based minerals. To combat acidic soil the farmers started using agricultural lime (calcium hydroxide) to alkalize the soil to grow vegetables; the vegetables now uptake inorganic nitrogen, phosphate, potassium, and calcium. Inorganic calcium and potassium are magnesium antagonists—every vegetable we eat depletes our magnesium reserves.There are over 45 different types of inorganic calcium used in many of the foods we consume. The list is beyond insane. They are used as flavor enhancers, intensifiers, anti-mold agents, anti-rope agents, buffers, neutralizing agents, stabilizers, suspending agents, foaming agents, gelling agents, thickeners, whipping agents, anti-caking agents, drying agents, dough conditioners, maturing agents, firming agents, nutrients, dietary supplements,To add insult to injury, there are over 38 different types of inorganic potassium used in many of the foods we consume. They are used as antimicrobial agents, buffers, neutralizing agents, preservatives, acids, acidifiers, emulsifiers, foaming agents, gelling agents, stabilizers, suspending agents, thickeners, whipping agents, bleaching agents, dough conditioners, maturing agents, oxidizing agents, dietary supplements, general purpose additives, antioxidant synergists, preservatives, etc.
When we have a magnesium deficiency, inorganic calcium builds up in the cells causing angina, arrhythmia, hypertension, headaches, and asthma. Magnesium is nature’s inorganic calcium channel blocker. Magnesium is also a potassium antagonist. Magnesium is our defense from inorganic calcium and potassium poisoning. When people talk of magnesium deficiency, they rarely talk about what kind of magnesium they are deficient in. There are many types of magnesium i.e. amino acid chelate, orotate, lactate, glycinate, malate, taurate, citrate, sulfate, chloride, aspirate, oxide, carbonate, hydroxide, bicarbonate, hydroxyl, organic magnesium, etc. In nature we came in contact with organic magnesium (which a plant converted into organic plant based magnesium) in the food we ate, magnesium bicarbonate in the water we drank, and magnesium bound to sodium chloride in sea salt. Magnesium bicarbonate is a bicarbonate ion (HC03) with the mineral magnesium hydroxide attached to it. Magnesium bicarbonate is an excellent base (buffers acids). The bicarbonate our body synthesizes will bind with magnesium hydroxide and produce magnesium bicarbonate.
Obviously, if we are constantly bombarded with inorganic calcium, potassium (magnesium antagonists), and sodium chloride, and up to 90% of Americans have a magnesium deficiency, and we want to buffer excess acids from our bodies, it is paramount to use the base our body needs–magnesium bicarbonate. When excess acids are buffered with magnesium bicarbonate we are more capable of resisting and chelating toxins. Magnesium bicarbonate protects us from the constant assault of noxious chemicals, heavy metals, and radiation exposure we are subjected to everyday in our water, food, and air.
Metabolic acidosis, magnesium deficiency, insufficient organic plant based minerals and a lack of bicarbonate salts are the main cause of most human disease. They are not only pivotal in preventing heart disease, they also prevent diabetes by helping the body properly regulate sugar metabolism. Excess vitamin D may lead to magnesium deficiency.
Without magnesium we could not produce energy, our muscles would be in a permanent state of contraction, and we could not adjust the levels of cholesterol produced and released into the blood stream.
Magnesium is the central element in chlorophyll and the basis of early life on the planet. It is crucial to more than 300 enzyme-driven biochemical reactions occurring in the body on a near constant basis. Enzymes are the basis of the body’s ability to function while supporting life. Without enzyme co-factors, including hormones and vital minerals, our health will spiral out of control. In fact even slight imbalances can chronically impact the body’s level of performance and health. Enzymes also play a vital role in the reactions that generate and use ATP, the fundamental unit of energy within the body’s cells.
Magnesium is the second most abundant element inside human cells and the fourth most abundant positively charged ion in the human body. Within the body’s cells, it serves literally hundreds of functions.
Magnesium is one of the most common co-factors in the body. Its presence is crucial to:
Glucose and fat breakdownProduction of proteins, enzymes and antioxidants such as glutathioneCreation of DNA and RNARegulation of cholesterol production
Magnesium and Heart Disease
The benefits of magnesium include the well-known decrease in ischemic heart disease and sudden death; prevention of platelet clumping (clot prevention), dilation of blood vessels, and improves the functioning of the heart muscle.
Magnesium calms the nerves. It mediates digestive processes; a lack of it is associated with many eating-related problems, including vomiting, indigestion, cramps, flatulence, abdominal pain, and constipation. When under stress, we use up much magnesium. Magnesium deficiency has been implicated in depression, diabetes, heart disease, migraines, and menopausal symptoms.
Hypermagnesemia (to much magnesium in the blood) is an uncommon clinical finding, and symptomatic hypermagnesemia is even less common. This disorder has a low incidence of occurrence, because the kidneys are able to eliminate excess magnesium (as long as the magnesium supplemented is in the correct form). As changes in extracellular and intracellular pH underlie the adverse effects of metabolic acidosis, the administration of all four bicarbonate salts—primarily magnesium bicarbonate—is the new mainstay of therapy. Bicarbonate physiology and chronic metabolic acidosis are entirely ignored for the host of medical problems we see today. Who would stop long enough to think deeply, and make the connection between drinking acidic water (acid rain), acid-producing diets, and over exercising with the destruction of the pancreas and a cascade of health problems?
Scientists already know a great deal about how acidification can disrupt the natural order, from the molecular level to the scale of living organisms:Enzymes, which catalyze vital reactions inside cells, are dependent on the acidity of the surrounding environment and are rendered less effective or totally inactive by increases in acid levels.Proteins, which comprise a significant part of the matter in all cells, undergo changes in geometry and function when altered.Organisms generally cannot reproduce and maintain themselves in optimal fashion unless their environment stays within a fixed range of acid/alkaline balance.
If we have metabolic acidosis and a magnesium deficiency—what is the result? Cancer, arthritis, decreased bone density, diabetes, heart disease, chronic fatigue, allergies, dry skin, weight gain or inability to lose weight, depression, inability to concentrate or focus, prone to colds and bronchitis, parasites, fungus, Candida, kidney stones, trouble with sleep patterns, just name it. At low pH levels (acidity), our immune system weakens and leads to dysfunction, allowing degenerative agents to thrive. One of the basic things that stand between you and perfect health is a magnesium deficiency your body’s pH—your basic metabolic body balance.
When magnesium bicarbonate enters body cells, the concentrations of bicarbonate ions inside body cells are increased. The bicarbonate derived from magnesium bicarbonate produces hydroxide ions (OH-) inside body cells, which neutralize the acid (H+) from carbon dioxide concentrations, ATP hydrolysis, and other sources. This occurs via a series of sequential and simultaneous reactions. Magnesium bicarbonate enters body cells and dissociates to increase bicarbonate ion concentrations inside body cells. Magnesium is not easily absorbed in the body unless first attached to transporting substance i.e. a bicarbonate ion (HCO3).
It is to be emphasized that magnesium bicarbonate assists in the maintenance of cell homeostasis. Intracellular homeostasis is very dependent on the “here and now;” it is very dependent on the immediate maintenance of ideal biochemical conditions.
Because magnesium bicarbonate decreases the accumulation of acids in body cells, it decreases the load for carbon dioxide elimination from the lungs and the acid elimination by the kidneys, this plays a role in homeostasis of the cell — ageing is greatly delayed.
The PristineHydro™ Solution = Electrolyte Balance™“Not Just minerals, but Natures Electrolyte Salts.”
Electrolyte Balance™ is nature’s way to neutralize excess acids, which allows the pancreas to balance (store and secrete) the internal pH of the body.
This unique formula is achieved through a patented brewing technology that re-creates the optimum circumstances that nature uses to properly prepare bicarbonate electrolyte salts and silicic acid, a bio-available form of silica. The benefits of Electrolyte Balance™ are as follows:Helps replenish the severe magnesium bicarbonate deficiency shared by up to 90% of all Americans.Facilitates calcium, potassium, and sodium voltage gated ion channels that allow magneto-electrical signaling in neurons and other excitable cells. Treating magnesium bicarbonate deficiency increases memory, focus, and deep relaxation.Magnesium bicarbonate helps protect cells from heavy metal poisoning, i.e. inorganic aluminum, mercury, lead, nickel, cadmium, fluoride, etc; noxious chemicals and radiation exposure.Magnesium bicarbonate helps with insomnia, headaches, and decreases inflammation in the body.All experiments with mammals have shown that magnesium bicarbonate increases life span up to 30%. This increase in life span is due to low carbon dioxide concentrations in body’s intracellular waters.With the addition of our “Electrolyte Balance™ Solution,” you can buffer the excess bad acids out of your body and allow the pancreas to balance (store and secrete) your pH. When your pancreas is unable to keep your pH balanced, you can manually balance your pH until your pancreas recovers.
Magnesium Bicarbonate Regulates our Electrolyte BalanceWithin every cell in the body, a proper balance of mineral content must be maintained. Magnesium’s role in the healthy balance (“homeostasis”) of important minerals such as calcium, sodium and potassium bicarbonate affects the conduction of nerve impulses, muscle contraction, and heart rhythms. Magnesium bicarbonate is more important than calcium, potassium, chloride, and sodium bicarbonate as it regulates all of them.
The body allows mineral ions to flow into and out of the cell from the extra-cellular fluid, depending on concentrations inside or outside the cell. Minerals, in their bicarbonate form, seek to equalize their concentrations by flowing through open membrane channels designed to allow movement of ions, water molecules, and small water-soluble compounds.
However, ideal levels for minerals inside and outside the cells are not equal, as minerals serve various purposes inside the body and the cells. To keep cells healthy, a distribution such as the following must be maintained.


Because of the tendency of bicarbonate salts to equalize across membranes, like water flowing toward the sea, the cell must actively move bicarbonate ions into or out of the cell, expending energy to create a healthy balance using special “exchange pumps”.
These mineral exchange pumps perform one of the most vital functions of the cell membrane, regulating the electrical action potential inside and outside of the cell, and maintaining homeostasis of minerals in the body. Without constant efforts by exchange pumps, cells would be flooded with calcium and sodium moving in, and potassium and magnesium moving out as they strived to achieve equilibrium.
One such exchange pump, known as the “sodium-potassium pump,” pumps sodium out of the cell in exchange for potassium. Embedded in the cell membrane, the sodium-potassium pump is activated by magnesium inside the cell.
Magnesium deficiency impairs the sodium-potassium pump, allowing potassium to escape from the cell, to be lost in the urine, potentially leading to potassium deficiency (hypokalemia). Those with a known potassium deficiency, therefore, often do not respond to treatment until magnesium deficiency is also corrected.
Similarly, magnesium’s role in calcium regulation is pivotal to its role in maintaining heart health. Magnesium is a known modulator of calcium, competing with calcium for entrance into cells and keeping many cellular processes in balance.
The effect of magnesium on blood vessels is one of dilation, whereas calcium promotes contraction.Magnesium is also thought to antagonize excess calcium promotion of blood clotting.
You can make smart changes in your lifestyle by drinking more PristineHydro™ water, adding Electrolyte Balance™ to the water you drink, taking Electrolyte Balance™ before bed and/or first thing in the morning, eating more alkaline-friendly foods, and using our natural PristineNutrition™ health products, and monitoring bicarbonate mineral reserves.
Magnesium bicarbonate is a complex hydrated salt that exists only in water under specific conditions. It does not occur in a solid form. Energized water containing all four electrolyte salts with extra magnesium bicarbonate is the best water on earth. Indeed, medical research has demonstrated that people live longer when they drink water that is acid free and appropriately mineralized. When consumed, magnesium bicarbonate rapidly enters body cells. This occurs because magnesium is an intracellular element and magnesium functions as a bicarbonate co-transporter into cells.
With its role in regulating the thousands of biochemical reactions that occur on an ongoing basis, sufficient magnesium is essential to achieving the delicate balance necessary to the body’s function. Protecting this delicate balance should be considered a fundamental goal in achieving optimal health and wellbeing. Electrolyte Balance™ contains laboratory grade alkalizing bicarbonate salts that will help buffer excess acids that overwhelm the pancreas, which allows the pancreas to recover its bicarbonate reserves and do its job— balance your pH. This gives your body the best chance of healing itself.
The Saliva pH Acid Challenge Test is the only valid test for chronic metabolic acidosis.
Salivary and urinary pH tests are not reliable because they fluctuate with what, when, or how much food we have eaten; what, when, and how much we drink, and/or how much we exercise, what kind of exercise, and when we exercised, and if we are fasting we become acidic. This tends to give varied pH readings.